Research
Genetic and Integrative Studies of Metabolic Dysfunction Associated Statotic Liver Disease
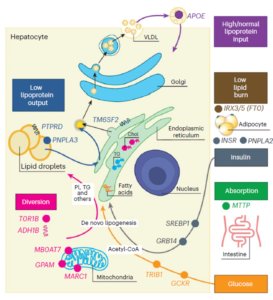
Metabolic dysfunction associated steatotic liver disease (MASLD) is a spectrum of disease that includes steatosis (fat) steatohepatitis (inflammation around that fat) and fibrosis/cirrhosis (scarring) in the liver. With the rise of obesity MASLD has reached epidemic proprotions affecting on average 30% of individuals in many countries. There are few effective treatments for MASLD presently. We have organize the Genetics of Obesity-Related Liver Disease (GOLD) Consortium in which we found that MASLD is about 25%–30% heritable. We found many SNPs that associate with hepatic steatosis and explain about 20% of the heritability of the trait and have differential effects on related metabolic traits including development of NASH/fibrosis, serum lipid and serum glucose. These studies identify subtypes of disease and are leading the way to precision diagnostics and therapeutics for these conditions. We have also identified environmental factors that multiplicatively interact with these variants and subtypes to cause disease which can be changes to mitigate disease risk.
Current Projects
1. Examine the effects of exonic and non coding variants on MASLD through meta analyses across ancestry groups in > 750K individuals
2. Fine mapping signals using cross ancestry analyses
3. Identifying rare variants that affect MASLD through sequencing
4. Examining the effect of MASLD associated variants on other traits through PheWAS analyses.
5. Examining gene environment interactions
6. Examining polygenic metabolic risk factor effects on MASLD
Genetic and Integrative Studies of Obesity and Anthropometric Traits
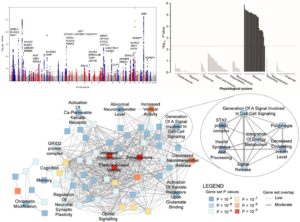 Obesity is a prevalent medical conditions that can lead to . It affects a sixth of the people worldwide, one third of Americans, and is associated with development of many other metabolic diseases including diabetes,cardiovascular disease and MASLD. There are few if any effective medical treatments for obesity. Through collaborative efforts in the Genetics of Anthropometric Traits Consortium (GIANT) we have have identified > 1000 loci that associate with body mass index (BMI, a measure of overall obesity) or Waist to Hip Ratio controlled for BMI (WHRadjBMI, a measure of abdominal obesity). We have also helped to develop methods to examine the enrichment of genes near our signals in tissues and in pathways. We find that many of the variants associated with BMI or WHRadjBMI are enriched for expression in brain or adipose tissue respectively and for enrichment in neuronal signaling, neuronal development, energy expenditure, appetite regulation for BMI and in adipose development and insulin signaling for WHRadjBMI. We have also carried out pleiotropy analyses where we have found subsets of genes affecting different groups of related metabolic disease similar to what we first reported for MASLD suggesting that we can identify subtypes of obesity. These studies are leading the way to precision diagnoses and treatments for obesity.
Obesity is a prevalent medical conditions that can lead to . It affects a sixth of the people worldwide, one third of Americans, and is associated with development of many other metabolic diseases including diabetes,cardiovascular disease and MASLD. There are few if any effective medical treatments for obesity. Through collaborative efforts in the Genetics of Anthropometric Traits Consortium (GIANT) we have have identified > 1000 loci that associate with body mass index (BMI, a measure of overall obesity) or Waist to Hip Ratio controlled for BMI (WHRadjBMI, a measure of abdominal obesity). We have also helped to develop methods to examine the enrichment of genes near our signals in tissues and in pathways. We find that many of the variants associated with BMI or WHRadjBMI are enriched for expression in brain or adipose tissue respectively and for enrichment in neuronal signaling, neuronal development, energy expenditure, appetite regulation for BMI and in adipose development and insulin signaling for WHRadjBMI. We have also carried out pleiotropy analyses where we have found subsets of genes affecting different groups of related metabolic disease similar to what we first reported for MASLD suggesting that we can identify subtypes of obesity. These studies are leading the way to precision diagnoses and treatments for obesity.
Current Projects
1. Identify genetic variants that associate with obesity across through larger more powered meta-analyses and through meta-analyses of non-European ancestries
2. Identifying variants that associate with extreme obesity and determining how those relate to variants that affect common obesity.
3. Identifying variants that affect fat mass.
Functional Studies of Obesity and MASLD
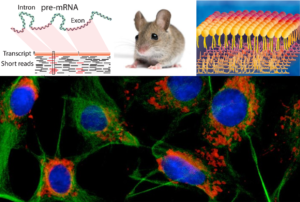 We have made cellular and mouse models of MASLD and obesity using lentiviruses, cas9/CRISPR technology and chemical/drug perturbations to define the mechanism of action of the genes that are implicated from GWAS analyses. We found that overexpressing or knocking down many of the gene implicated from our obesity or MASLD analyses affect serum biomarkers, insulin and glucose sensitivity, fat disctribution, tissue histology, changes in genes expression (using RNAseq) and lipid balance (using lipidomics). We are also screening for supressors of pathological cellular effects using cas9/CRISPR technology or through chemical/drug screening with high content readouts. These studies help to better define subtypes of disease and create new precision diagnostics and therapeutics for obesity and MASLD.
We have made cellular and mouse models of MASLD and obesity using lentiviruses, cas9/CRISPR technology and chemical/drug perturbations to define the mechanism of action of the genes that are implicated from GWAS analyses. We found that overexpressing or knocking down many of the gene implicated from our obesity or MASLD analyses affect serum biomarkers, insulin and glucose sensitivity, fat disctribution, tissue histology, changes in genes expression (using RNAseq) and lipid balance (using lipidomics). We are also screening for supressors of pathological cellular effects using cas9/CRISPR technology or through chemical/drug screening with high content readouts. These studies help to better define subtypes of disease and create new precision diagnostics and therapeutics for obesity and MASLD.
Current Projects
1. Assess the effects on genes implicated from GWAS though knockdown in mouse models and assess effects on serum biomarkers, tissue histology, gene expression, and lipid balance.
2. Overexpress/knock down genes implicated by GWAS studies in liver, adipose and muscle cell lines using cas9/CRISPR technology and assess their effects on metabolic endpoints using high content image analysis
3. Screen for suppressors of pathologic endpoints in cell culture using cas9/CRISPR or chemical/drug probes
Genetics of Human Traits in Cohorts and Biobanks
 We are interested in how genetics influences obesity and related human diseases in the real world. We are examining genetic and phenotype data from the UK Biobank, AllOfUs, TOPMed and Michigan Genomics Initiative to determine the genetic basis of many human disease and traits. We are examining the pleiotropic effect of genetic changes on serum biomarkers, histology, metabolites, protein levels and disease endpoints. Results from this work will reveal causes of human disease, biomarkers of disease subtypes, and new treatment targets.
We are interested in how genetics influences obesity and related human diseases in the real world. We are examining genetic and phenotype data from the UK Biobank, AllOfUs, TOPMed and Michigan Genomics Initiative to determine the genetic basis of many human disease and traits. We are examining the pleiotropic effect of genetic changes on serum biomarkers, histology, metabolites, protein levels and disease endpoints. Results from this work will reveal causes of human disease, biomarkers of disease subtypes, and new treatment targets.
Current Projects
1. PheWAS analyses of genes implicated in obesity and MASLD analyses.
4. GWAS of human traits
5. Structure function of genes using rare variant analysis, functional studies and outcomes.
6. Using machine learning/AI to predict phenotypes and outcomes